




Nomenclature and Numbering Convention
Many of the programmes and techniques we use have different methods of naming and numbering atoms. As a standard, atoms of all molecules studied should be numbered as follows:
- Number atoms according to IUPAC standard
- IUPAC full instructions
- ChemSketch download, which can automatically generate a name for the molecule, and although it has a function to renumber, it is not very good.
- Keeping the atoms in the same order, renumber within each element from 1 with no gaps in the sequence.
- If two or more atoms have the same number
- decide which is the major part of the structure (using the systematic name)
- keep the numbering of the major part of the structure
- number additional groups in sequence of which atom of the main
structure they are attached to.
In most cases, a ring would be the main structure, but in some cases a phenyl group would be considered a side group.
- If two identical groups are numbered with the same numbers (e.g. 1, 2, 3, 4, 5, 6 & 1', 2', 3', 4', 5', 6'), number all the atoms in one group, and then all the atoms in the other group in the same order
- If you have two molecules, number all the atoms in one molecule, and then all the atoms in the other molecule.
- Put atoms in numerical order, grouped by element
- Order element blocks as follows:
- Carbon
- All other atoms in ascending atomic number
- Hydrogen
- Keep a record of the systematic atom numberings, and how they correspond with the numbers you have used. Call the text file number.system and upload it to the data portal with the overview files at the end of the search.
Example - pyridine-2-one
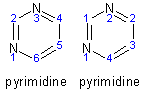
- The molecule was drawn into ChemSketch, numbering automatically generated, and its name generated to give the numbering and name on the left.
- The atoms were renumbered in the manner on the right
- Carbon atoms had their numbers reduced to consecutive numbers starting from 1.
- Nitrogen atoms were renumbered in the same manner.
- Hydrogens would be numbered starting from that attached to the lowest numbered carbon atom.
Example - 2-Amino-3-(4-hydroxy-phenyl)-propanal
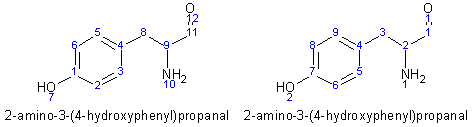
- The molecule was drawn into ChemSketch, numbering automatically generated, and its name generated to give the numbering and name on the left.
- The atoms were renumbered in the manner on the right
- The molecule has been named as a propanal, so the aldehyde is the main group. Therefore, its numbering is retained and the carbon atoms in the phenyl ring follow sequentially.
- The nitrogen atom is numbered 1.
- The aldehyde oxygen is numbered 1 and the ring oxygen 2, as they are attached to carbon atoms in this sequence.
- The hydrogens attached to carbon atoms are numbered first, in sequence of increasing number of the atom to which they are attached. Then, as nitrogen is next in increasing atomic number, the hydrogen atoms attached to nitrogen are numbered next. Finally the hydroxy hydrogen is numbered.
Example - progesterone
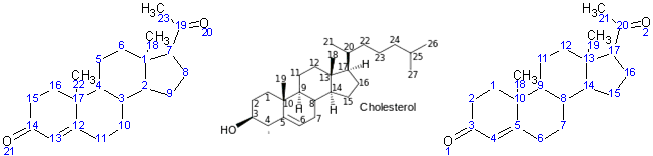
- The molecule was drawn into ChemSketch, and numbering automatically generated as on the right. A systematic name could not be produced as there are more than three rings.
- The numbering of cholesterol in the Templates window of ChemSketch or on the Wikipedia webpage for progesterone gives the numbering in the middle, and the IUPAC systematic name from Wikipedia is Pregn-4-ene-3,20-dione, so we need to ensure that the numbers are as consistent as possible with this (as in the numbering in the middle)
- The atoms were renumbered in the manner on the right
- Carbons in the ring structure kept their numbering.
- Carbons on side chains were numbered next, starting with the chain attached to the lowest numbered ring carbon (n.b. this meant swapping the numbering of the 18 and 19 carbon atoms)
- Oxygen atoms are numbered in increasing order of the carbon atom to which they are attached.
- Hydrogens would be numbered in the same manner, starting with those attached to the lowest numbered carbon atom.
© UCL Chemistry Department 2022. This page was last updated on 17 August, 2022. If you have any problems with this page please email the WebMaster